Useful optimization of electrical cell-substrate impedance sensing (ECIS) utilizing human corneal epithelial cells
[ad_1]
Three-dimensional bio-impedance evaluation
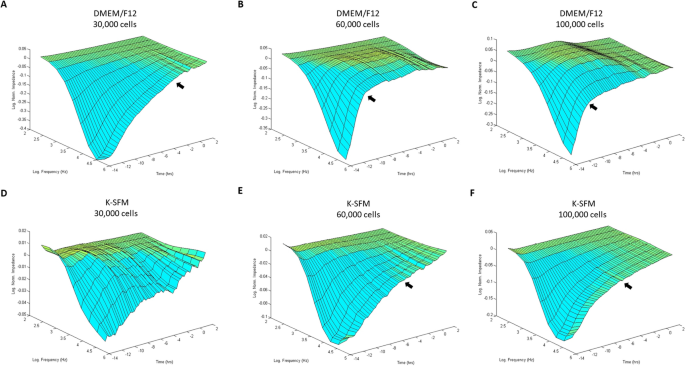
Bio-impedance evaluation of HUCLs was carried out to match two completely different cell tradition media (DMEM/F12 and Okay-SFM) at three completely different cell densities (30,000, 60,000 and 100,000 cells per properly) as proven in Fig. 1A–F. HUCLs fashioned a mature confluent barrier as indicated by a plateau in impedance (Z) represented as log normalized values on the y-axis within the 3D mannequin. As such, HUCLs grown in DMEM/F12 media in any respect three seeding densities (A–C) fashioned a mature confluent barrier sooner than cells equally grown in Okay-SFM media (D–F). Moreover, three-dimensional representations of normalized impedance throughout HUCLs as a operate of each time and log frequency confirmed DMEM/F12 at a density of 60,000 was most optimum for barrier maturation (B). Likewise, at a 60,000-seeding density, the logarithmic development curve reached a plateau (time to confluency) after 4–6 h with DMEM/F12 (B) in comparison with 8–10 h with Okay-SFM (E). Thus, indicating that HUCLs grown within the supplemented media extra effectively kind an epithelial monolayer than cells equally grown in unsupplemented media.
Barrier operate of HUCLs monitored by real-time bio impedance evaluation utilizing an AC frequency scan. HUCLs have been seeded on a 96W1E + ECIS array. Three-dimensional representations of the log of normalized impedance (y-axis) as a operate of each log frequency of the alternating-current (AC) (y-axis) and time (z-axis). Cells grown in DMEM/F12 and Okay-SFM are proven for 30,000 (A,D), 60,000 (B,E) and 100,000 (C,F) cell seeding densities. Arrows point out begin of plateau and approximate time to confluency.
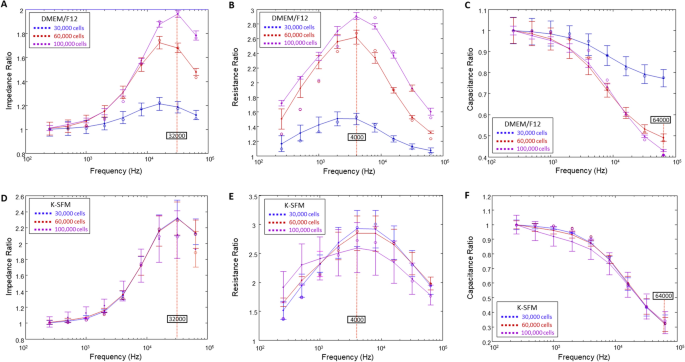
Subsequent, we aimed to dissect the affect of DMEM/F12 and Okay-SFM media on impedance (Z), resistance (R) and capacitance (C). When cells are challenged with an AC, pure R and C are created, which collectively outcome within the general impedance, Z. Nonetheless, to find out the optimum frequencies to make use of in evaluating every of those parameters, frequency dependence spectra have been first measured as proven in Fig. 2. The frequency dependence of variables Z, R, and C for cells grown in DMEM/F12 on the three cell densities at T = 15 h are proven in panels A–C, respectively. Panels D–F show the identical info for HUCLs grown in Okay-SFM at T = 15 h. Right now level, each teams have already fashioned confluent monolayers and are anticipated to have fashioned an intact barrier. Ratios of cell to cell-free measurements are plotted in opposition to frequency for impedance (A,D), resistance (B,E), and capacitance (C,F). As offered in Fig. 2, the impedance spectrum confirmed a attribute frequency of 32 kHz, offering the best potential vary for group comparability of cells grown in DMEM/F12 (A) and Okay-SFM media (D). However, we noticed that 4000 Hz produces the utmost resistance in each DMEM/F12 and Okay-SFM media (Fig. 2B,E, respectively). Additional, capacitance ratios displayed that an optimum minimal response was achieved at 64 kHz for each DMEM/F12 (Fig. 2C) and Okay-SFM media (Fig. 2F). Nonetheless, Okay-SFM confirmed overlap between the three cell densities with larger commonplace deviations than DMEM/F12, thus indicating potential suboptimal situations for HUCLs rising within the Okay-SFM media.
Willpower of optimum AC frequencies utilizing frequency dependence spectra. Knowledge are offered as ratios of cell to cell-free measurements (y-axis) versus frequency (x-axis) measured at 15 h. Tracings are proven for impedance ratios with a most response at 32 kHz (A,D), resistance ratios with a most response at 4000 Hz (B,E), and capacitance ratios with a minimal response at 64 kHz (C,F) from HUCLs grown in DMEM/F12 (A–C) and Okay-SFM (D–F), respectively. Knowledge proven are the imply ± SEM; n = 5/group.
Impedance measurements
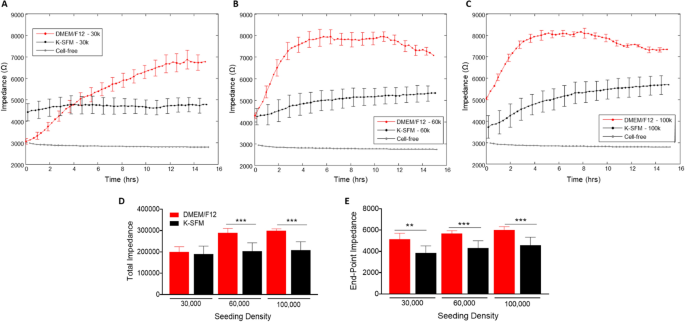
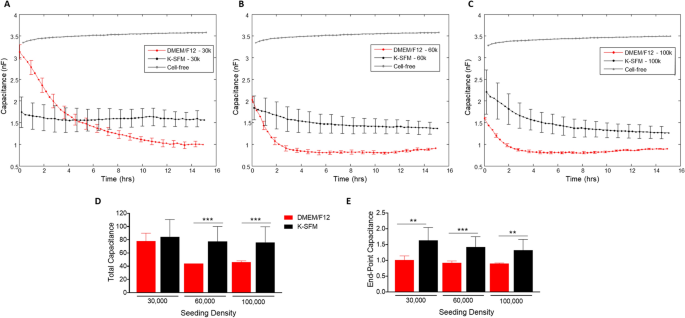
Impedance (capacitive reactance) measurements, as proven in Fig. 3A–E, calculated at a excessive frequency of 32 kHz present info as to when the cell monolayer is in place and confluent. That is mirrored by the plateau within the impedance measurements. Cells grown in DMEM/F12 reached the plateau part at 14–15 h for the 30,000-seeding density, and at ~ 4 h for each 60,000- and 100,000-seeding densities. Whereas HUCLs seeded on the similar densities however grown in Okay-SFM didn’t show as distinct a plateau part, indicative of poor HUCL spreading, nonetheless reached confluency at ~ 8 h for 60,000 cells and 6–8 h for 100,000 cells. This development is additional illustrated in each complete (Fig. 3D) and endpoint (Fig. 3E) impedance measurements generated at 32 kHz; impedance values for HUCLs grown in DMEM/F12 have been considerably greater when in comparison with Okay-SFM media. These impedance measurements point out that the HUCLs grown within the DMEM/F12 are capable of kind and keep a powerful and confluent monolayer over time. Thus, indicating that investigation of HUCL barrier formation needs to be carried out utilizing supplemented DMEM/F12 media rather than the classically used Okay-SFM media.
Actual-time monitoring of HUCL impedance in DMEM/F12 versus Okay-SFM media. Impedance of HUCLs versus time, measured at an AC frequency of 32 kHz for 30,000 (A), 60,000 (B) and 100,000 (C) seeding densities is proven. Bar graph illustration of complete impedance (D) and end-point impedance (E) evaluating DMEM/F12 versus Okay-SFM. Knowledge proven are the imply ± SEM; n = 5/group. **p ≤ 0.01 and ***p ≤ 0.001.
Resistance measurements
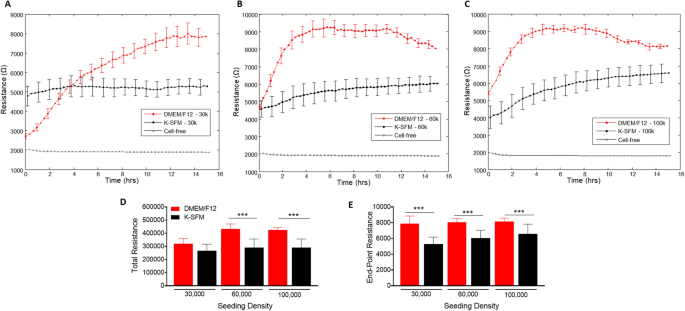
Resistance measurements taken at a low frequency gives perception into the barrier formation and performance. Determine 4A–C present resistance measurements generated at 4000 Hz from HUCLs seeded on the three completely different cell densities and grown within the two completely different tradition media. Barrier formation is indicated by the plateau part in every resistance profile. HUCLs grown in supplemented DMEM/F12 reached the very best resistance (9000 Ω) at 60,000 (B) and 100,000 (C) seeding densities. Nonetheless, HUCLs cultured in Okay-SFM reached a much less distinct “plateau part” with a most resistance of ~ 6000 Ω. The full and endpoint resistance values proven in Fig. 4D and E, respectively, have been generated out to a most of 16 h as decided by the barrier formation plateaus for each teams of media. Whole resistance values have been considerably greater in DMEM/F12 versus Okay-SFM at seeding densities of 60,000 and 100,000 cells. Moreover, endpoint resistance measurements confirmed that each one three cell densities grown in DMEM/F12 have been considerably greater in comparison with Okay-SFM cells, indicating the formation of tighter and stronger epithelial cell obstacles when grown in DMEM/F12. Collectively, these outcomes help that the optimum development, barrier formation, and sustaining situations for HUCLs are greatest carried out within the DMEM/F12 media. With out the supplementation, as indicated by HUCLs grown in Okay-SFM, “mature” obstacles aren’t as properly fashioned, thus offering additional proof for the usage of supplemented DMEM/F12 media when learning corneal epithelial operate in vitro. These developments have been noticed when carried out to later time factors, as properly, as proven at 70 h in Supplemental Fig. 1.
Actual-time monitoring of HUCL resistance in DMEM/F12 versus Okay-SFM media. Resistance of HUCLs versus time, measured at an AC frequency of 4000 Hz for 30,000 (A), 60,000 (B) and 100,000 (C) cell seeding densities is proven. Bar graph illustration of complete resistance (D) and end-point resistance (E) evaluating DMEM/F12 versus Okay-SFM. Time = 0 h denotes time of inoculation. Knowledge proven are the imply ± SEM; n = 5/group. ***p ≤ 0.001.
Capacitance measurements
As with the resistance measurements described above, the expansion traits of HUCLs have been noticed within the real-time formation of confluent cell layers and measured as capacitance (Fig. 5A–E). Cells grown within the supplemented DMEM/F12 media displayed extra environment friendly cell spreading in any respect seeding densities in comparison with cells grown in Okay-SFM. On the 30,000 seeding cell density (A), cells grown in DMEM/F12 reached a confluent monolayer between 8 and 10 h. HUCLs seeded at 60,000 and 100,000 cells grown within the DMEM/F12 fashioned a confluent monolayer between 2 and 4 h (B and C). Whereas HUCLs at both 30,000 or 60,000 seeding densities grown in Okay-SFM exhibited a lot much less environment friendly cell spreading. Cells grown in Okay-SFM have been capable of set up a confluent layer; nonetheless, it took longer at 5–6 h as mirrored by capacitance. To additional illustrate the variations between DMEM/F12 and Okay-SFM within the formation of a confluent layer, complete and endpoint capacitance measurements are additionally proven. Whole capacitance (Fig. 5D) was considerably decrease at 60,000 and 100,000 cell seeding densities for DMEM/F12 in comparison with Okay-SFM. As proven in Fig. 5E, endpoint capacitance for all three seeding densities was considerably decreased in DMEM/F12, as properly. Due to the inverse relationship between capacitance and cell spreading, it’s indicated that the DMEM/F12 media higher helps the expansion, spreading and formation of a confluent mobile layer in comparison with the classically used Okay-SFM rising situations.
Actual-time monitoring of HUCL capacitance in DMEM/F12 versus Okay-SFM media. Capacitance of HUCLs versus time, measured at an AC frequency of 64 kHz is proven for 30,000 (A), 60,000 (B), and 100,000 (C) cell seeding density. Whole capacitance (D) and end-point capacitance (E) evaluating DMEM/F12 versus Okay-SFM are represented by bar graphs. Knowledge proven are the imply ± SEM; n = 5/group. **p ≤ 0.01 and ***p ≤ 0.001.
Mathematical modeling of the R knowledge—Rb, α and Cm
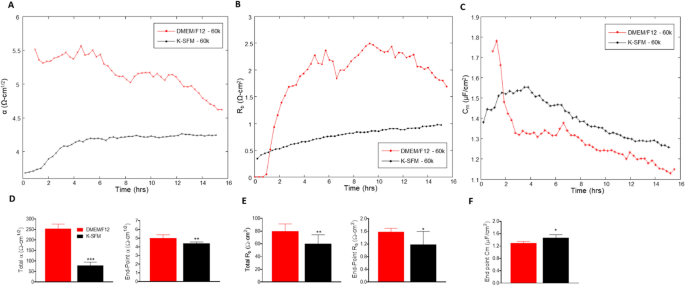
The ECIS software program has the flexibility to mannequin the impedance into parameters that distinguish between cell–cell (Rb) and cell–matrix (α) adhesions, in addition to membrane capacitance (Cm). Rb is the resistivity of cell–cell contacts to the present movement. α measures the impedance contributions arising from the cell–electrode junctions. Due to this fact, the contribution of Rb, α, and Cm to the noticed modifications in earlier experiments was calculated by becoming a mathematical mannequin developed by Giaever and Keese8. Rb, α, and Cm values have been decided from HUCLs on the 60,000-cell seeding density grown in DMEM/F12 in comparison with Okay-SFM media and are offered in Fig. 6A–F.
Mathematical modeling of α, Rb, and Cm for HUCLs grown in DMEM/F12 versus Okay-SFM media. Modeled parameters, α (A), Rb (B), and Cm (C) have been traced over 15 h for cells seeded at 60,000. Time = 0 denotes time of inoculation. Bar graphs characterize complete and end-point values from DMEM/F12 versus Okay-SFM media for α (D) and Rb (E); end-point solely is proven for Cm (F). Knowledge proven are the imply ± SEM; n = 5/group. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
The constructed parameter α, indicating the energy of interplay between the cells with the basal substrate, is greater in cells grown in DMEM/F12 in comparison with Okay-SFM all through the complete time course (Fig. 6A). These outcomes mixed with complete and endpoint α measurements (Fig. 6D), that are additionally considerably greater for HUCLs grown in DMEM/F12 in comparison with Okay-SFM, point out that cells grown within the DMEM/F12 media create stronger mobile attachments to the basal substrate. These knowledge may additionally contribute to the general variations seen within the resistance values between HUCLs grown in DMEM/F12 versus Okay-SFM.
Moreover, Rb values, which mirror paracellular barrier energy, have been greater in HUCLs cultured in DMEM/F12 media when in comparison with HUCLs grown in Okay-SFM media (Fig. 6B). This noticed enhance in barrier operate is additional demonstrated by corresponding complete and endpoint Rb values (Fig. 6E), the place HUCLs grown in DMEM/F12 displayed considerably greater Rb values than Okay-SFM media. Along with the α worth, the truth that HUCLs grown in DMEM/F12 displayed greater Rb values in comparison with the cells grown in Okay-SFM signifies that stronger cell–cell interactions are additionally enjoying an underlying function within the general variations noticed in resistance.
Cm or the capacitance of the cell membrane, proven in Fig. 6C, is indicative of temporal alterations in membrane thickness and composition. Moreover, Cm measurements are used to find out if variations in capacitance are solely attributable to modifications in electrode protection or are a operate of microvariations within the apical membrane buildings. Whole Cm just isn’t offered since confluent monolayers are required to mannequin this parameter, which didn’t persistently happen at earlier timepoints (< 6 h) for cells grown in Okay-SFM. In consequence, solely end-point Cm is proven, which is considerably decrease in HUCLs grown in DMEM/F12 in comparison with Okay-SFM (Fig. 6F). Due to this fact, the interpretation from the information is that the variations in Cm are attributable to variations in electrode protection and never membrane construction.
Mobile morphology
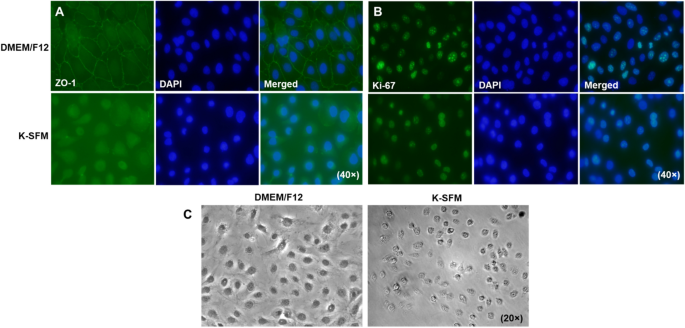
To correlate our practical research with morphological variations, ranges of ZO-1, a part of the tight junction complicated required for paracellular signaling, have been decided qualitatively by immunostaining (Fig. 7A). HUCLs grown in DMEM/F12 exhibit a steady linear sample of ZO-1 staining alongside cell–cell borders with DAPI stained nuclei. This attribute cobblestone like sample was not noticed in cells grown in Okay-SFM. In reality, cells appeared extra rounded with fewer cell–cell interactions and little or no constructive ZO-1 staining. HUCLs have been additionally stained for the proliferation marker, Ki-67, as proven in Fig. 7B. Each teams confirmed comparable staining patterns indicating comparable proliferation charges between the 2 varieties of media. Nonetheless, phase-contrast microscopy (Fig. 7C) reiterated what was noticed with the ZO-1 staining. HUCLs grown in DMEM/F12 show a polygonal form which are tightly joined with little intercellular area. Regardless of comparable cell numbers, these grown in Okay-SFM didn’t seem squamous or cuboidal, however as a substitute had a spherical look with only a few cell–cell contacts.
Visible observations of HUCLs cultured in DMEM/F12 and Okay-SFM. Ranges of ZO-1 (A) and Ki-67 (B) have been assessed by immunofluorescence after 5 days of culturing in both DMEM/F12 or Okay-SFM media. ZO-1 and Ki-67 are proven in inexperienced with nuclei of cells counterstained with DAPI proven in blue. Photographs are proven at 40 × . Section-contrast microscopy (C) photos of HUCLs grown in each DMEM/F12 and Okay-SFM are proven at 20 × .
Response to wounding stimulus
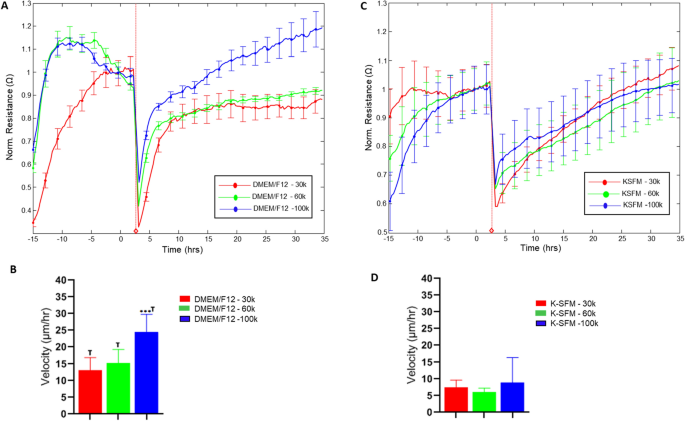
To analyze additional into the practical influence of the noticed variations in tradition situations, HUCLs have been preliminarily assessed for potential variations in response to wounding, as proven in Fig. 8. Charges of restoration following wounding have been decided as velocity of cell migration (derived from normalized resistance values) for cells cultured in DMEM/F12 (A, B) and Okay-SFM (C, D) for every seeding density over time till 100% of the normalized resistance previous to wounding was reached. Variations in restoration time have been noticed between the 2 media for all seeding densities with cell velocities roughly 50% decreased in Okay-SFM in comparison with DMEM/F12. This differential response to a typical stimulus comparable to wounding means that culturing situations have an effect that extends past the preliminary culturing response by HUCLs.
Wound therapeutic response of HUCLs grown in DMEM/F12 versus Okay-SFM media. Normalized resistance of HUCLs versus time, measured at an AC frequency of 4000 Hz for 30,000, 60,000 and 100,000 cell seeding densities is proven for DMEM/F12 (A) and Okay-SFM (B). Bar graph illustration of cell velocity of migrating cells (C,d) for each teams over time. Time = 0 h denotes time of wounding. Knowledge proven are the imply ± SEM; n = 5/group. ***p ≤ 0.001; † p ≤ 0.01 between DMEM/F12 and Okay-SFM teams.
[ad_2]
Source_link