Optimization, purification, and characterization of xylanase manufacturing by a newly remoted Trichoderma harzianum pressure by a two-step statistical experimental design technique
[ad_1]
Screening of great medium constituents for xylanase manufacturing
The rows in Desk 2 symbolize the twelve completely different experiments. The information obtained from the PBD runs point out a large variation in xylanase exercise from 9.8 to 68.7 U/ml throughout the twelve runs. This variation demonstrated that the impact of the medium and tradition circumstances on the manufacturing of xylanase was important (p < 0.05). The R2, or coefficient of dedication, is the proportion of response variance that may be ascribed to the mannequin slightly than a random error44. Based on Xie et al.45, R2 ought to be not less than 90% for a mannequin to suit effectively. The dedication coefficient (R2) signifies that the unbiased variables had been chargeable for 97 p.c of the pattern variance in xylanase output, and simply roughly 3% of the general variation was not defined by the mannequin. The larger the correlation between experimental and anticipated values, the nearer R (correlation coefficient) is to 1. The worth of R (0.97) indicated that the experimental information and the theoretical values predicted by the mannequin equation had been in shut settlement. As indicated in Desk 5, the p-value was used to confirm the importance of every of the coefficients. The incubation interval (X2), pH (X3), and wheat bran (X5) had been all proven to have a major (p < 0.05) impact on xylanase exercise. The Pareto chart of standardization (Fig. 1) confirmed that these three elements considerably influenced xylanase manufacturing (p < 0.05), as they crossed the p-line. Nonetheless, the opposite unbiased elements (p > 0.05) had been usually thought of insignificant.
There’s a 97% likelihood that the mannequin explains the measured variations in response. The magnitude and course of the issue coefficient within the equation make clear the affect of the six variables for xylanase manufacturing. The upper magnitude indicated a big impact on the response. The corresponding response of xylanase exercise was expressed by way of the next regression Eq. (3) derived from the Unstandardized Beta values (Desk 6):
$${textual content{Y}} = {textual content{X}}_{{1}} + {textual content{X}}_{{2}} + {textual content{X}}_{{3}} + {textual content{X}}_{{4}} + {textual content{X}}_{{5}} + {textual content{X}}_{{6}} ,$$
(3)
$${textual content{Y }} = { 2}0.{58 } – , 0.{textual content{47X}}_{{1}} + { 4}.{textual content{28X}}_{{2}} + { 1}.{textual content{51X}}_{{3}} {-} , 0.{textual content{16X}}_{{4}} + { 23}.{textual content{99X}}_{{5}} + { 4}.{textual content{56X}}_{{6}} ,$$
the place Y is outlined as the height space, X1 refers back to the incubation temperature, X2 is the incubation time, X3 is the pH, X4 is the agitation, X5 is the wheat bran and X6 is the ammonium sulphate.
Optimization of great variables for xylanase manufacturing
Field Behnken design
A complete of 16 runs had been carried out to find out the circumstances for optimum xylanase manufacturing by T. harzianum. A matrix was run with the three important variables that emerged from the PBD experiments. The outcomes for the BBD runs (Desk 4) present that the bottom exercise of 27.38 U/ml was obtained underneath zero-level circumstances (5 days, pH 5.0, and 1% wheat bran) in run 15 whereas run 8 resulted within the highest xylanase exercise of 153.80 U/ml underneath the next circumstances: 6 days of incubation, pH 5.0, and 1.2% wheat bran. This was considerably and markedly (over four-fold)_higher (p ≥ 0.05) than the very best enzyme actions obtained throughout OFAT (38.50 U/ml). Lengthy et al.21 confirmed an analogous however decrease affect of optimized parameters on xylanase manufacturing (174.46–266.70 U/ml) by Trichoderma orientalis. Utilizing the quadratic equation, the anticipated values had been decided (Desk 4). The R2 or coefficient of dedication (0.9647, near 1) confirmed the validity of the mannequin, i.e., that 96.47% of the variability of the response could be expressed by the mannequin. The worth of the coefficient of adjusted dedication, adjusted R2, was 0.9112 confirming that the precise values had been near the anticipated values46,47. The correlation was confirmed by plotting the precise worth curve as a perform of the anticipated values (Fig. 2) which reveals the factors distributed across the regression line. Determine 2 reveals that the precise response values agreed effectively with the anticipated response values, thus the anticipated xylanase manufacturing is inside the limits of the experimental elements. Due to this fact, the mannequin is taken into account of adequate high quality46 with a 96.47% likelihood that it explains the measured variations in response.
Most xylanase manufacturing (153.80 U/ml) by the T. harzianum pressure occurred in BBD run 8 underneath acidic circumstances (pH 5.0), the upper wheat bran (1.2%), and a 5-day incubation interval. Calmly decrease exercise could be noticed for run 12 (116.74 U/ml) the place the incubation interval was 5 days, the wheat bran was 1.2% and the pH was 6.0. Even decrease however comparable enzyme actions had been obtained for runs 6 (101.32 U/ml) and seven (103.37 U/ml) the place both the incubation time (4 or 6 days) or wheat bran (0.8 or 1.2%) was at their low or excessive ranges, respectively in comparison with run 8 the place each these parameters had been at their excessive ranges (6 days and 1.2%). This can be as a result of presence of two isoforms which might be maximally produced underneath acidic circumstances. The presence of isoforms requires completely different durations of incubation for maximal xylanase exercise and numerous wheat bran concentrations. Within the presence of xylan, most microorganisms can produce a number of forms of xylanases. Fungi are well-known for producing a variety of xylanases (as much as 30 a number of kinds)5,48. Zhang et al.49 reported that three xylanase isoforms had been produced by Aspergillus fumigatus. A number of types of xylanases differ in stability, catalytic effectivity, absorption, and exercise on substrates50. Okafor et al.51 remoted a pressure of Penicillium chrysogenum PCL501 from wooden wastes and located that after 4 days of fermentation, wheat bran produced the very best xylanase exercise of 6.47 U/ml. Abdel-Sater et al.52 obtained most xylanase manufacturing from T. harzianum after 8 days of fermentation whereas, Thomas et al.53 achieved most xylanase manufacturing in 4 days of fermentation by an Aspergillus sp.
The manufacturing of a number of types of xylanases could be influenced by many elements, together with the presence of varied alleles of the identical gene, variable mRNA processing, proteolytic digestion put up secretion, and post-translational modifications equivalent to glycosylation and autoaggregation54. As a result of xylanases have various catalytic efficiencies, the manufacturing of a number of xylanases is especially useful for the entire hydrolysis of hemicellulosic substances55. Typically, xylanase manufacturing is instantly proportional to the period of the fermentation time as much as a sure degree after which decreases, thus, incubation time impacts xylanase manufacturing by fungi56.
Second-order regression and prediction
The second-order regression equation gives the xylanase exercise produced by the T. harzianum pressure as a perform of incubation time (X2), pH (X3), and wheat bran (X5) which could be offered within the following Eq. (4):
$${textual content{Y}} = { 44}.{91} – 0.00{textual content{4X}}_{{2}} + 0.0{textual content{12X}}_{{3}} + {42}.0{textual content{9X}}_{{5}} – 0.00{textual content{4X}}_{{{22}}} + 0.0{textual content{12X}}_{{{32}}} + {42}.0{textual content{9X}}_{{{52}}} + {textual content{X}}_{{2}} {textual content{X}}_{{3}} + {textual content{X}}_{{2}} {textual content{X}}_{{5}} + {textual content{ X}}_{{3}} {textual content{X}}_{{5}} ,$$
(4)
the place Y is the height space, X2 is the incubation time, X3 is the pH and X5 is the wheat bran focus. The statistically insignificant parameters (p > 0.05) and their interactions had been omitted from the equation. The mannequin constants and coefficients had been generated utilizing the unstandardized beta values.
ANOVA and Pareto chart
The “Lack of match p-value” (Desk 7 ) was insignificant because the p-value was larger than 0.05, nonetheless, literature reveals this p-value (> 0.05) is taken into account acceptable57. Based on Bezerra et al.58, important regression and a non-significant lack of match current within the mannequin had been well-fitted to the experiments. Primarily based on this, the regression equation could be validated59. ANOVA was carried out to find out the p-values (Desk 7). This confirmed the mannequin, the linear and sq. phrases for X2 (Incubation time), and the interplay between X3 (pH) and X5 (Wheat bran) in addition to the linear phrases of X3 (pH) to be important because the p-values had been 0.00001, 0.0001, 0.00005, 0.02042 and 0.01137, respectively. The Pareto chart of standardization histogram graph (Fig. 3) additionally confirmed that Incubation time (X2, X22), the interplay between pH and wheat bran (X3, X5), and pH (X3) was important (p < 0.05), because it crosses the p-line.
Interplay of variables
The connection between the parameters and the responses could be understood by learning the three-dimensional (3D) response floor plots for xylanase exercise generated from the quadratic mannequin. The 3D response floor plot will also be used to find out the optimum degree of every variable for xylanase exercise (Figs. 4, 5, 6). Whereas sustaining different variables at their optimum degree, the Z-axis (referring to xylanase exercise) versus any two variables was constructed within the response floor plot.
Determine 4a,b illustrate the mixed results of incubation time and pH xylanase exercise will increase at a excessive pH and shorter incubation time. Determine 4b illustrates the contour plot, which reveals that top enzyme exercise was obtained on the shortest (4 days) and longest interval (6 days) of incubation in acidic (4.0–6.0) circumstances. Yadav et al.29, reported comparable pH circumstances for optimization of xylanse manufacturing from Anoxybacillus kamchatkensis NASTPD13.
Determine 5a,b present that xylanase manufacturing is instantly proportional to incubation time and wheat bran. This could possibly be as a result of larger ranges of degradation of xylan current within the wheat bran by T. harzianum. In Fig. 5b, it’s obvious that the xylanase exercise is highest at excessive concentrations of wheat bran with the shortest (4 days) and longest interval (6 days) of incubation. Earlier research confirmed the time course throughout the OFAT strategy, being beneficial at 4 days and 6 days of incubation with the optimum being at 5 days33. The RSM plots correspond with the OFAT outcomes, because the plots present the very best xylanase exercise obtained at excessive wheat bran between 4 and 6 days. Concurrently, it was highlighted by Beg et al.60 that wheat bran might successfully induce larger xylanase manufacturing by Aspergillus awamori. Li et al.61 additionally reported the significance of the substrate concentrations for xylanase manufacturing by A. awamori. The info talked about right here, correspond to the reviews by Cui and Zhao62, as they point out that the enzymes, that are concerned in substrate degradation, had been usually largely inducible. These had been shaped solely when the substrate it correlates with, was current within the nutrient salt resolution.
Determine 6 reveals the very best xylanase exercise obtained with excessive wheat bran and all the pH vary examined. Nonetheless, at decrease wheat bran concentrations, larger xylanase exercise could be noticed on the pH extremes examined (at roughly pH 4 and pH 6). Determine 6b illustrates, at excessive wheat bran focus and over a large pH vary with the very best exercise obtained on the highest pH and wheat bran focus. The interplay between the pH and wheat bran (130 U/ml) and between incubation time and wheat bran (130 U/ml) had the very best impact on xylanase exercise in comparison with the interplay between incubation time and pH (120 U/ml).
The research efficiently demonstrated a notable improve in enzyme exercise utilizing the statistically designed experiments in comparison with OFAT. It was additionally demonstrated that a number of types of xylanase had been produced (isoforms) based mostly on variations within the development and media circumstances. Primarily based on Desk 4, there might probably be 5 completely different isoforms. Excessive xylanase exercise was noticed for runs 4, 6, 7, 8, and 12. Supplementary Fig. 6 representing these RSM runs signifies the presence of isoforms by a number of zones of clearance on the substrate native PAGE gels.
A number of types of xylanases with completely different pH optima could possibly be useful for animal feed enchancment13. Xylanase is used to scale back the viscosity of the feed and enhance the absorption of vitamins within the digestive tract of animals. The enzymes could possibly be utilized earlier than the pelleting course of, which operates between pH 4.0 and 6.0 thus requiring enzymes which might be energetic inside this pH vary. Most xylanases reported thus far are optimally energetic within the acidic or impartial pH vary. Xylanases with acidic pH optima might probably even be helpful for purposes containing waste, as a technique of waste administration, and as a feedstock for fermentable sugars63.
Scaled-up fermentation in optimized circumstances for additional research
The xylanase enzyme was produced on a bigger scale for additional research. The enzyme was produced at pH 5.0 for six days of incubation and with 1.2% wheat bran. The enzyme exercise was decided to be able to examine the actions to the smaller scale manufacturing. The enzyme exercise obtained was 152.78 U/ml which was just like the enzyme manufacturing on a smaller scale (153.80 U/ml).
Purification of xylanase from the T. harzianum isolate and zymography
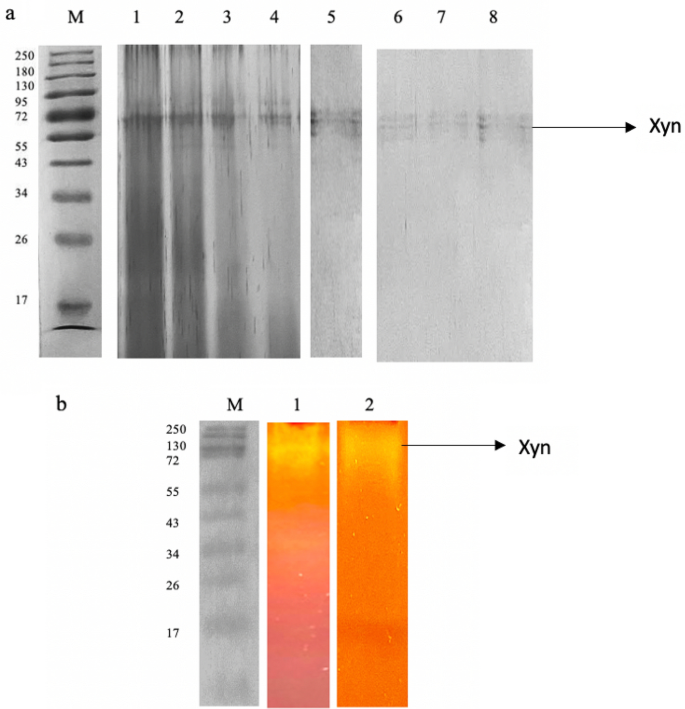
The xylanase from T. harzianum was purified utilizing ammonium sulphate precipitation, dialysis, and chromatographic strategies together. Desk 8 summarizes the purification phases. The enzyme was fractionated with the next ammonium sulphate saturations: (0–19%, 20–29%, 30–39%, 40–49%, 50–59%, 60–69% and 70–79%). The 70–79% saturation fraction resulted in considerably excessive xylanase exercise with a restoration of 20.31% enzyme exercise. The 50–59%, 60–69%, and fractions additionally confirmed comparatively excessive restoration of enzyme exercise (18.73%, and 17.48%, respectively) whereas 10.42% enzyme was recovered within the 40–49% fraction (Desk 8) subsequently these fractions had been additional studied to substantiate in the event that they had been isoforms. The energetic fractions had been then dialyzed at 4 °C in a single day to take away the salts, and the enzyme was loaded onto DEAE Sephadex for additional purification. A 0–2 M sodium chloride focus gradient was used to elute the certain protein. Xylanase exercise was measured in each certain and unbound protein fractions. The first peak eluted at 0.5 M sodium chloride and the corresponding fraction had a particular exercise of 254.62 mol/mg, and a 2.52-fold purity. Moreover, a single band with a molecular weight of 72 kDa was evident on SDS-PAGE gels of the purified enzyme (50%) (Fig. 7a). The opposite ammonium sulphate fractions (60–79%) even have the identical molecular weight protein (72 kDa). To evaluate the exercise/purity, the purified xylanase was subjected to zymogram evaluation by substrate native-PAGE (1% beechwood xylan). The xylanolytic exercise of the enzyme was indicated by clear zones within the gel after Congo-red staining (Fig. 7b). Purified preparations of enzymes are a requisite for his or her utility in addition to elucidating their fundamental traits and mechanisms. Primarily based on the excessive molecular weight of the purified enzyme, it may be tentatively inferred that it might belong to the GH10 household since enzymes belonging to this household characteristic a bigger molecular weight63. Enzymes are additionally categorised based mostly on their catalytic reactions. Primarily based on the sequence similarities of amino acids, xylanases are categorised into glycosyl hydrolase (GH) households 10 (GH10) and 11 (GH11)2. Household GH10 incorporates xylanases of excessive molecular mass (> 30 kDa) with a (β/α)8 barrel construction and acidic pI values, whereas GH11 embody are the low-molecular-weight endoxylanases that are divided into alkaline pI and acidic pI xylanases2.
A 12% SDS PAGE (a) and Native substrate-PAGE (b) evaluation of purified xylanase. 12% SDS PAGE (cropped) represents Lane M: Molecular weight marker (Thermoscientific, USA), 1–4: 50, 60, 70, and 80% ammonium sulphate fractions (Coomassie-stained), and 5–8: Purified xylanase from Trichoderma harzianum (Xyn). Native substrate-PAGE (cropped) represents Lane M: Molecular weight marker (Thermoscientific, USA), 1: 50% Ammonium sulphate fraction displaying zone of clearance, and Lane 2: Purified xylanase (Xyn) from Trichoderma harzianum on native substrate gel displaying zone of clearance. The unique gels are offered in Supplementary Figs. 1–5.
Characterization of xylanase
pH optimum and stability
The enzyme exercise is tremendously affected by pH as a result of substrate binding and catalysis are depending on the cost distribution of each the substrate and the enzyme molecules. The response pH was adjusted to 4.0–10.0 with numerous buffers as described above. The optimum pH of T. harzianum xylanase is pH 6.0 with an exercise of 40 U/ml (Fig. 8a). The enzyme is pretty steady at pH 6.0 and stays energetic (Fig. 9) retaining > 70% of its exercise over 4 h. Souza et al.64 reported that the xylanase from Thermoascus aurantiacus expressed in E. coli confirmed optimum exercise and stability at an analogous pH. Yadav et al.29 reported that xylanase from Anoxybacillus kamchatkensis NASTPD13 confirmed excessive exercise between pH 6.0 to 9.0 and at pH 6.0, the enzyme retained 71% of its exercise over 24 h. The purified 60–79% ammonium sulphate fraction was additional confirmed to include the identical protein as that purified within the 50% fraction because it displayed the identical pH optimum and dimension (pH 6.0) (Fig. 8a). Thus, the purified fractions of the 50–79% ammonium sulphate fractions could be mixed to extend the yield (%).
Optimum temperature and thermal stability
The experiment was carried out at completely different response temperatures starting from 40 to 79 °C to seek out the optimum temperature of the xylanase. The best exercise of xylanase was noticed at 65 °C (Fig. 8b). Thermal stability information illustrated in Fig. 9 reveals that the enzyme retained > 70% exercise at 65 °C for 4 h. The same end result was reported by de Oliveira Simões et al.65. Nonetheless, in that research, the enzyme was subjected to therapy for twenty-four h and was steady for 1 h. The purified 60–79% ammonium sulphate fraction contained the identical purified protein because the 50% fraction, with the identical molecular weight, pH and temperature optima obtained (65 °C) (Fig. 8b). This confirms that these fractions will not be isoforms of the xylanase produced. Nonetheless, the form of the curve for the 50% ammonium sulphate fraction is completely different from the opposite fractions, which appear to point out an optimum slightly than a broad bell form.
Some great benefits of enzymes that choose excessive temperatures are well-known as a result of the solubility of the reagents and merchandise is elevated, the viscosity is decreased, and the mass switch charge is larger66. When searching for enzymes for industrial makes use of, stability, and exercise at excessive temperatures are extremely fascinating.
Impact of steel ions and inhibitors
The consequences of 8 steel ions (Ca2+, Co2+, Fe2+, Mg2+, Mn2+, Zn2+, Okay+, and Na+) at a ultimate focus of two mM and 10 mM on xylanase exercise had been decided (Desk 9) on the optimum pH and temperature (6.0 and 65 °C). Enzyme exercise was barely elevated by 2 mM Mn2+, Okay+, and Na+ (101.11–101.77 U/ml) whereas the enzyme exercise was barely however considerably larger with 10 mM Ca2+, Co2+, Fe2+, Mg2+, Zn2+ (104.27–110.89 U/ml) (p ≥ 0.05) and thus, these ions act as cofactors for the enzyme. Most enhancement was noticed for Fe2+ (10.88%) adopted by Mg2+ (9.43%) and Zn2+ (8.43%) at 10 mM. Fu et al.43 reported comparable findings for xylanase from Trichoderma sp. TPS-36.
Inhibitory results had been noticed for Fe2+ (15.29%), Mg2+ (3.44%), Zn2+ (5.95%) at 2 mM and Na+ (1.24%) at 10 mM, nonetheless, this inhibition of xylanase was weak (< 50%). Co2+ and Ca2+ had no impact on xylanase exercise (100%) at both concentrations.
Fu et al.43 additionally reported weak (< 50%) inhibition of xylanase with the identical ions and that Co2+ and Ca2+ had no impact on xylanase exercise (100%) at both concentrations.
Substrate specificity of purified xylanase
To find out the substrate specificity of the xylanase for polysaccharide degradation, potential substrates, together with birchwood xylan, beechwood xylan, wheat arabinoxylan (soluble and insoluble), xylan from Larchwood, CMC and Avicel had been examined underneath optimum circumstances (pH 6.0 and temperature 65 °C) utilizing the purified xylanase. Greater hydrolytic exercise was noticed for the xylans from beechwood, birchwood, and Larchwood in comparison with wheat arabinoxylan (Desk 10). The xylanase most actively degraded birchwood xylan (174.07%), adopted by Larchwood xylan (131.03%), and offered the bottom exercise in the direction of wheat arabinoxylan (soluble 70.54% and insoluble 46.62%). The purified xylanase completely hydrolyzed xylans, with no exercise on CMC and Avicel. This urged that xylanase’s substrate-binding area has a excessive affinity for xylans from softwood (birchwood and beechwood)67. This is likely to be as a result of variations in xylan polymer buildings and the presence of reactive teams on the floor which might be extra readily certain. The purified xylanase exhibited important hydrolytic exercise on the varied xylan substrates, indicating that it is likely to be categorised as an endo-1,4-xylanase68.
Kinetic evaluation
The Michaelis fixed, Okaym, could also be decided by measuring the substrate focus at half the utmost velocity. Okaym is a continuing that continues to be mounted for each given enzyme and substrate mixture. Consequently, a low Okaym improves the enzyme’s affinity for the substrate69 The focus vary of the substrate underneath investigation was 1–20 mg/ml, the research revealed Okaym and Vmax had been 5.56 mg/ml and 1052.63 µmol/min/mg (Fig. 10). The worth of Okaym is inside the vary of fungal xylanases reported in literature (0.14–14 mg/ml). Raj et al.70 obtained comparable values (4.96 mg/ml and decrease Vmax 402 µmol/mg/ml) for xylanase from alkaliphilic Bacillus licheniformis. Fu et al.43 reported excessive Vmax (1250 µmol/min/mg) just like this research. As a result of xylanase has a excessive Vmax worth and a low Okaym worth, it has a excessive affinity for the substrate, beechwood xylan, and may catalyze it extra effectively and shortly than different substrates. Xylanases from Caldicoprobacter algeriensis sp. nov. pressure TH7C1T had been proven to have excessive selectivity for beechwood xylan42.
[ad_2]
Source_link